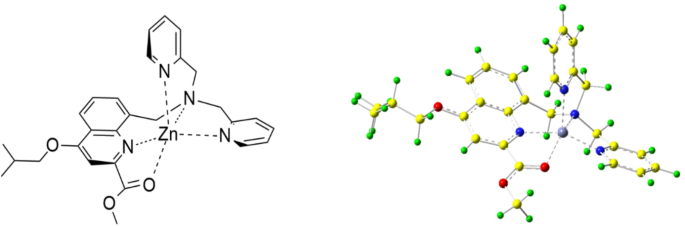
A novel simplified structural design as an artificial enzyme for efficient hydrolysis of PNPA
Murakami, Y., Kikuchi, J. I., Hisaeda, Y. & Hayashida, O. Artificial enzymes. Chem. Rev. 96, 721–758 (1996).
Google Scholar
Wallimann, P., Mattei, S., Seiler, P. & Diederich, F. New cyclophanes as initiator cores for the const- ruction of dendritic receptors: Host-guest complexation in aqueous solutions and structures of solid-state inclusion compounds. Helv. Chim. Acta. 80, 2368–2390 (1997).
Google Scholar
Benkovic, S. J. & Hammes-Schiffer A perspective on Pnzyme catalysis. Science 301, 1196 (2003).
Google Scholar
Hammes-Schiffer, S. & Benkovic, S. J. Relating protein motion to catalysis. Annu. Rev. Biochem. 75, 519 (2006).
Google Scholar
Dong, Z. Y., Luo, Q. & Liu, J. Q. Artificial enzymes based on supramolecular scaffolds. Chem. Soc. Rev. 41, 7890–7908 (2012).
Google Scholar
Merlo, C., Dill, K. A. & Weikl, T. R. Φ values in protein-folding kinetics have energetic and structural components. Proc. Natl. Acad. Sci. U S A. 102, 10171–10175 (2005).
Google Scholar
Resmini, M. Molecularly imprinted polymers as biomimetic catalysts. Anal. Bioanal Chem. 402, 3021–3026 (2012).
Google Scholar
Wei, H. & Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 42, 6060–6093 (2013).
Google Scholar
Wu, J. et al. Nanomaterials with Enzyme- like characteristics (Nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48, 1004–1076 (2019).
Google Scholar
Buhleier, E., Wehner, W. & Vögtle, F. Cascade-chain-like and nonskid-chain-like syntheses of molecular cavity topologies. Synthesis-Stuttgart 2, 155–158 (1978).
Google Scholar
Tomalia, D. A. et al. A new class of polymers: starburst-dendritic macromolecules. J. Polym. 17, 117–132 (1985).
Google Scholar
Lombardo, V., Bonomi, R., Sissi, C. & Mancin, F. Phosphate diesters and DNA hydrolysis by dinuclear Zn(II) complexes featuring a disulfide Bridge and H-bond donors. Tetrahedron 66, 2189–2195 (2010).
Google Scholar
Gao, H. et al. Dinuclear Zn(II) complex catalyzed phosphodiester cleavage proceeds via a concerted mechanism: A density functional theory study. J. Am. Chem. Soc. 9, 2904–2915 (2011).
Google Scholar
Pengo, P., Polizzi, S., Pasquato, L. & Scrimin, P. Carboxylate-Imidazole cooperativity in dipeptide-functionalized gold nanoparticles with esterase-like activity. J. Am. Chem. Soc. 127, 1616–1617 (2005).
Google Scholar
Pengo, P., Baltzer, L., Pasquato, L. & Scrimin, P. Substrate modulation of the activity of an artificial nanoesterase made of peptide-functionalized gold nanoparticles. Angew Chem. Int. Ed. 46, 400–404 (2007).
Google Scholar
Manea, F., Houillon, F. B., Pasquato, L., Scrimin, P. & Nanozymes Gold-nanoparticle-based transphosphorylation catalysts. Angew Chem. Int. Ed. 43, 6165–6169 (2004).
Google Scholar
Pasquato, L., Rancan, F., Scrimin, P., Mancin, F. & Frigeri, C. N-methylimidazole-functionalized gold nanoparticles as catalysts for cleavage of a carboxylic acid ester. Chem. Commun. 20, 2253–2254 (2000).
Google Scholar
Gea, A. et al. Solid-supported synthesis of highly functionalized tripodal peptides with flexible but preorganized geometry: towards potential Serine protease mimics. Eur. J. Org. Chem. 71, 4135–4146 (2006).
Google Scholar
Rossi, P. et al. An azacrown-functionalized peptide as a metal ion based catalyst for the cleavage of a RNA-model substrate. Biopolymers 55, 496–501 (2000).
Google Scholar
Bernhardt, P. V. et al. An approach to more accurate model systems for purple acid phosphatases (PAPs). Inorg. Chem. 54, 7249–7263 (2015).
Google Scholar
Bosch, S., Comba, P., Gahan, L. R. & Schenk, G. Dinuclear zinc(ii) complexes with hydrogen bond donors as structural and functional phosphatase models. Inorg. Chem. 53, 9036–9051 (2014).
Google Scholar
Bosch, S. et al. Selective coordination of gallium(iii), zinc(ii), and copper(ii) by an asymmetric dinucleating ligand: a model for metallophosphatases. Chem. Eu J. 21, 18269–18279 (2015).
Google Scholar
Comba, P., Gahan, L. R., Mereacre, V., Hanson, G. R. & Zajaczkowski-Fischer, M. Spectroscopic characterization of the active FeIIIFeIII and FeIIIFeII forms of a purple acid phosphatase model system. Inorg. Chem. 51, 12195–12209 (2012).
Google Scholar
Mendes, L. L. et al. Metallohydrolase biomimetics with catalytic and structural flexibility. Dalton Trans. 45, 18510–18521 (2016).
Google Scholar
Norberto, Douglas, R. et al. Second-Sphere effects in dinuclear Fe(III) zn(II) hydrolase biomimetics: tuning binding and reactivity properties. Inorg. Chem. 57, 187–203 (2018).
Google Scholar
Comba, P. et al. Monoesterase activity of a purple acid phosphatase mimic with a cyclam platform. Chemistry. Chem. Eur. J. 18, 1700–1710 (2012).
Google Scholar
Jayasinghearachchige, V. M. et al. Elucidating the roles of distinct chemical factors in the hydrolytic activities of hetero- and homonuclear synthetic analogues of binuclear metalloenzymes. ACS Catal. 13, 3131–3147 (2023).
Google Scholar
Pereira, C., Farias, G., Maranha, F. G., Castilho, N. & Peralta, R. A. Guanidine- and purine-functionalized ligands of FeIIIZnII complexes: effects on the hydrolysis of DNA. J. Biol. Inorg. Chem. 24, 675–691 (2019).
Google Scholar
Akkaya, E. U. & Czarnik, A. W. Synthesis and reactivity of cobalt(III) complexes bearing primary-side and secondary-side cyclodextrin binding-sites – A Tale of 2 CDS. J. Am. Chem. Soc. 110, 8553–8554 (1988).
Google Scholar
Briggs, G. E. & Haldane, J. B. S. A note on the kinetics of enzyme action. Biochem. J. 19, 338–339 (1925).
Google Scholar
Lnznaster, M. et al. J. Biol. Inorg. Chem. 10, 319–332 (2005).
Google Scholar
Mitić, N. et al. The catalytic mechanisms of binuclear metallohydrolases. Chem. Rev. 106, 3338–3363 (2006).
Google Scholar
Neves, A. et al. An unprecedented FeIII(µ-OH)ZnII complex that mimics the structural and functional properties of purple acid phosphatases. J. Am. Chem. Soc. 129, 7486–7487 (2007).
Google Scholar
link



:strip_icc()/BHG-What-Is-Modern-Landscape-Design-8yvZqRI3KPCAXsiCinicm6-861b7909a5c44ec5b0236c10751a240d.jpg)